(858) 224-9100
What Is HPLC Band Broadening
Band Broadening in HPLC: A Simple Overview
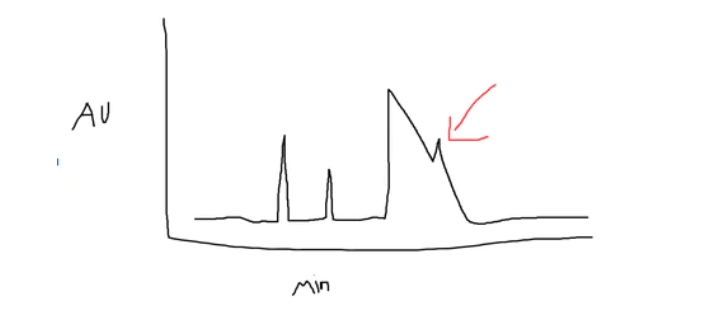
I’m sure in the lab when you are analyzing your result you probably have gotten something like this before…

Ahh yep, the good ‘ol HPLC band broadening problem – when your results aren’t as accurate as you’d like them to be and you potentially have to figure out how to make your peaks look sharper.
That’s why in this guide we are going to go over HPLC band broadening and how to minimize these peaks.
What is Band Broadening?
Band broadening is when your graph peaks on your chromatogram flatten out.
So instead of looking like those nice, crispy separated peaks, they flatten out and they start to bleed into other peaks.
This is reflected in the analyte (the chemical component you’re analyzing) in your HPLC column.
So why is this a problem?
This is a problem because it means that the sample amount will not be accurate. When your results aren’t accurate, this will mess up all your calculations and results because you don’t have the right data.
This is an immense problem because, with chromatography, the general goal is to separate your components and analyze them. If the graph shows it’s not separating properly, then it’s going to be a problem.
Not only will you not be able to quantify your results properly, but there might be a mixing of your analytes, causing your data to be inaccurate.
The Van Deemter Effect

In 1956, Van Deemter created a formula for the major effects of band broadening below:
H = A + B/u + C*u
Where this is what each part of the equation stands for:
- H= Plate height
- A = Eddy diffusion (Multipath diffusion)
- B = longitudinal diffusion (Occur throughout nature)
- C = mass transfer
- u = Linear flow rate (Through the column)
Eddy Diffusion (Multipath Diffusion)

Eddy Diffusion, or multipath, is one factor that affects HPLC Band Broadening. This is because depending on how your analytes go through the column, this can affect when they arrive at the end, broadening the peak.
Think of it like racing across a school courtyard with trees and gardens in the way. If all the kids run through different obstacles in different directions with the same goal line at the end, then different kids will arrive at different times.
It’s the same situation with the analytes in your HPLC column. Depending on the path your analytes take, it can take shorter or longer for the same analyte to reach the end of the HPLC column.
Also, in theory, A can be 0, but in reality, you will probably have to deal with it to some extent (since the real world isn’t like a textbook.)
Longitudinal diffusion

The next part of the equation we have to be aware of is longitudinal diffusion. This occurs in nature, but over time, the particles start to slowly drift apart and fill up the column.
If we look at the equation (B/u) we notice that if the flow rate is incredibly slow, that will make the number smaller in the denominator making that part of the equation huge. The reason for this is that when we give the particles more time in the column, it spreads apart where the same analyte will arrive at different times, making the peaks broaden.
If the flow rate is incredibly fast, then the particles are more likely to stay together in one place since there isn’t much time spent in the column and the peaks will be sharper.
It should be noted – you wouldn’t want to just increase the flow rate and blast it because that can cause pressure issues for your HPLC which can cause an entire set of other issues.
Mass transfer

The next part of the equation we have to consider is the mass transfer. As we can see in the equation with C*u, we notice that as the flow rate is faster, C becomes bigger (making the peaks broaden more).
While if the slow rate is slower, then there’s more of a chance that the number will be smaller.
The reason this happens depends on how your analytes react with your guard column. You will want to have a good surface area in your column because it creates more opportunities for more separation and cleaner retention times. For example, if your particles or beads are porous, then chances are not every analyte will react in the same way.
Some particles will not have much reactivity and will go straight through the column with little resistance, while others may travel along the edges. The reason this may be a problem is that if you increase the flow rate, chances are the same analytes will arrive at different times, broadening the peaks. However, if we slow down the flow rate, it gives the analyte a better chance to stay together so that the analytes can roughly come out at the same time giving you that nice pointy peak.
How Do We Minimize Band Broadening?
Let’s talk about how we can minimize hand broadening for HPLC so that you can get better data.
The first thing you can help with multipath diffusion is to get well-packed columns that are sourced from a good company. You’d also like it with smaller stationary particles and uniform size and distribution to keep things consistent.
Longitudinal Diffusion
- You would want to have moderate flows (not too slow or too fast) as being too slow can increase band broadening and too fast can hurt your column with pressure problems.
- You would want a column length that is reasonable (not too long or too short) for proper separation.
- Use minimal tubing required with a smaller diameter to ensure that the particles don’t spread out too far apart.
Mass Transfer
- You would want to use smaller stationary phase particles.
- You might consider heating the columns (depending on which column you’re using) to help keep nonporous and porous bound particles uniform
- You want to use a lower to moderate flow rate to ensure the analytes are not separating too far apart.
Conclusion
I hope you found this useful to learn more about HPLC band broadening and how to reduce it to help you get much more accurate data. If you have any questions, you can always contact us at info@filtrous.com.


Leave a comment